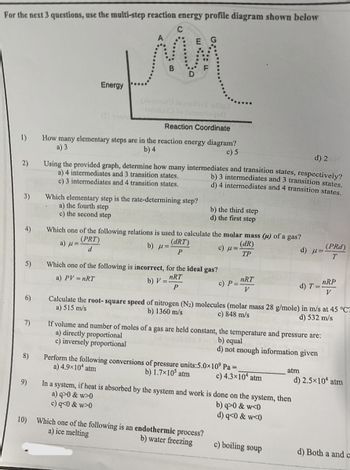
40 multistep reaction energy profile
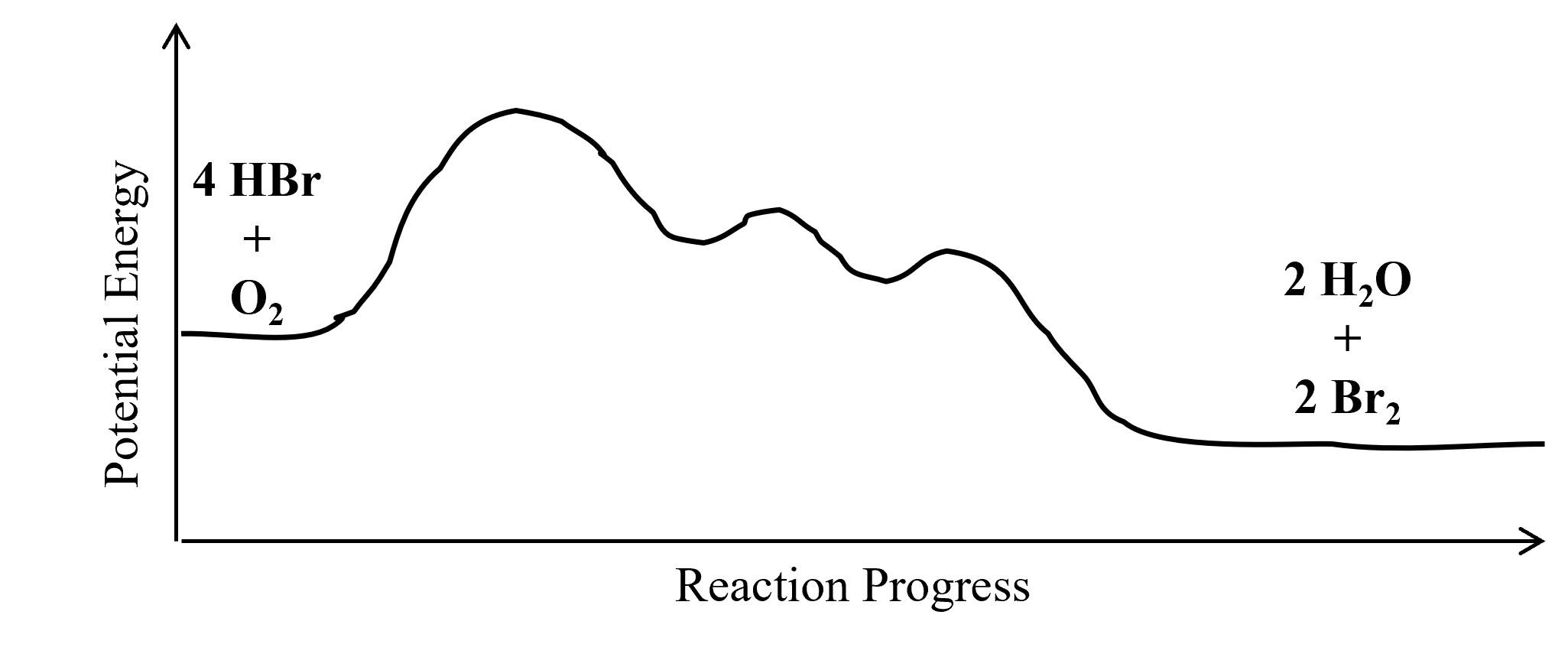
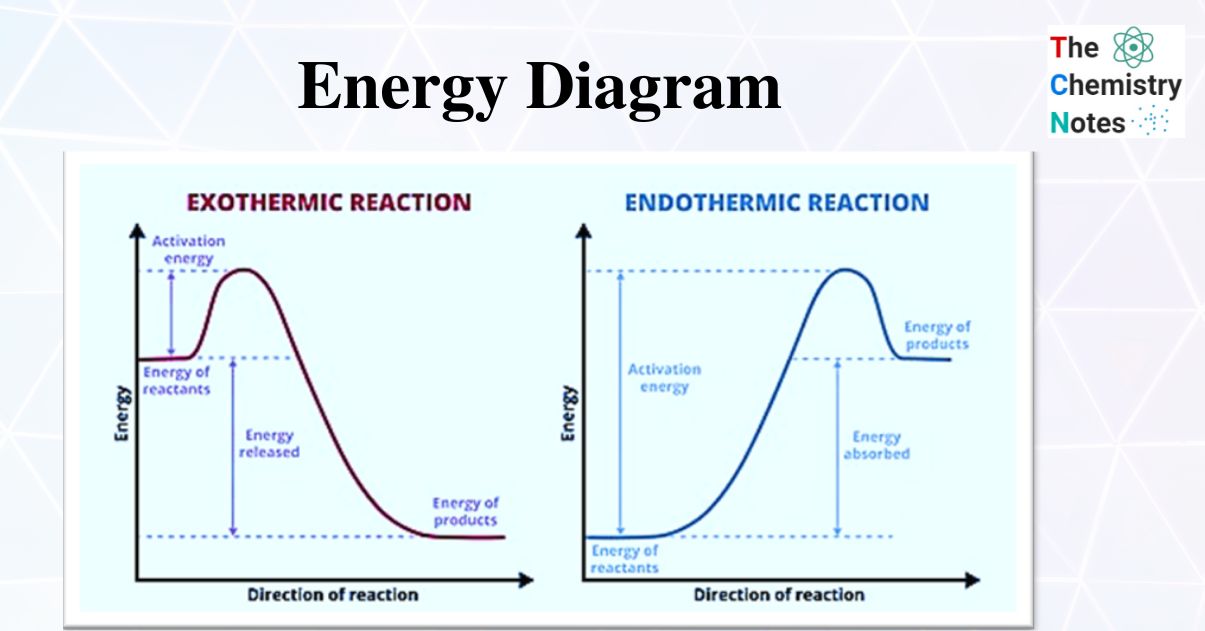
› explanations › chemistryEnergy Profile: Definition, Diagram, Reaction | StudySmarter Energy Profile Chemical Analysis Formulations Instrumental Analysis Pure Substances Sodium Hydroxide Test Test for Anions Test for Metal Ions Testing for Gases Testing for Ions Chemical Reactions Acid-Base Reactions Acid-Base Titration Bond Energy Calculations Decomposition Reaction Electrolysis of Aqueous Solutions Electrolysis of Ionic Compounds › multistep-reaction-energy-profileMultistep Reaction Energy Profile | StudyAPChemistry In addition, the energy of the reactants won't always be higher than that of the products. This is an exothermic reaction, as heat energy is released. We will cover this more in the next unit, which is Thermodynamics. That's basically it for the Multistep Reaction Energy Profile.
› notes › ap_chemistryMultistep Reactions - Softschools.com The exact mechanism by which a multistep reaction occurs can be determined by observations such as. Direct evidence of an intermediate. The rate law by which the reaction proceeds. The energy diagram of a two-step reaction is shown below.
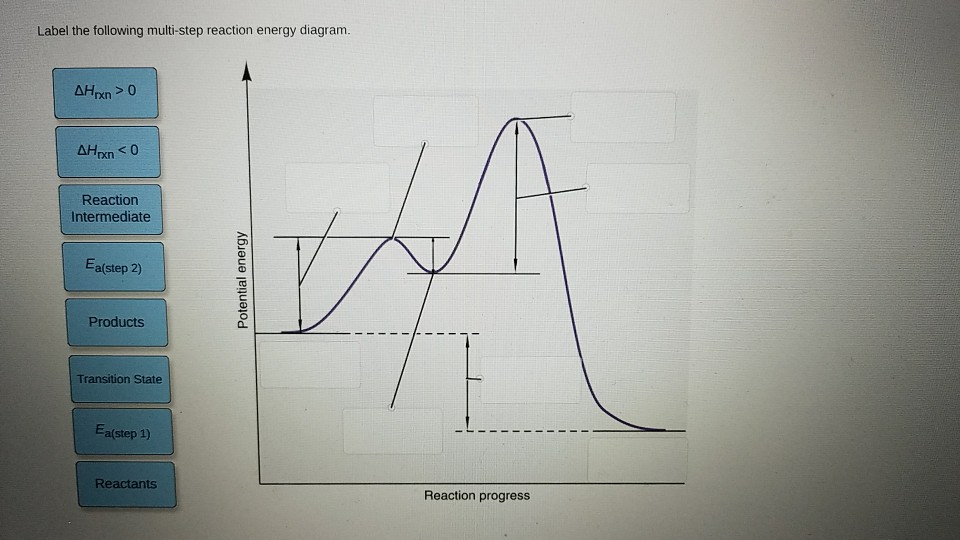
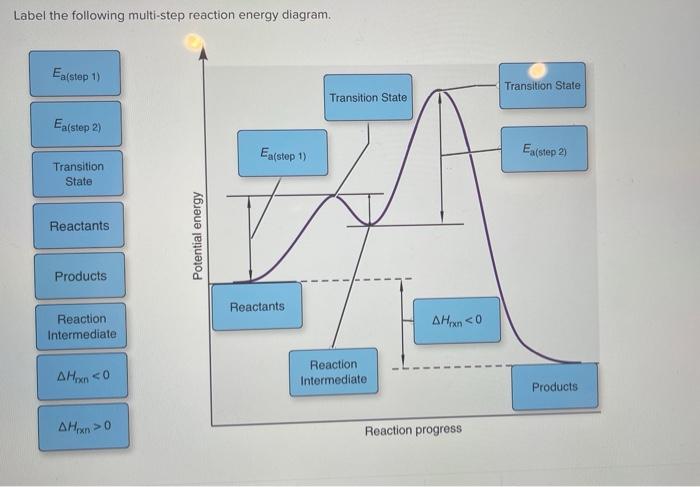
Multistep reaction energy profile
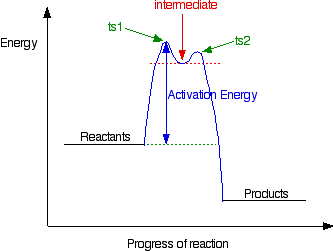
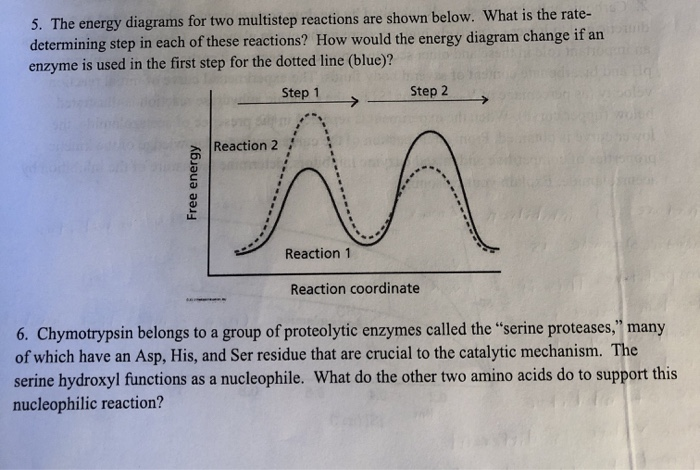
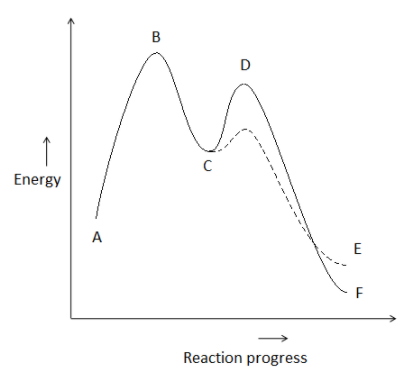
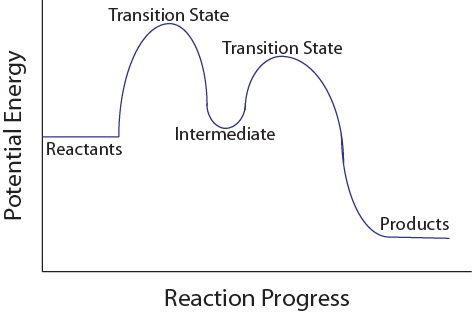
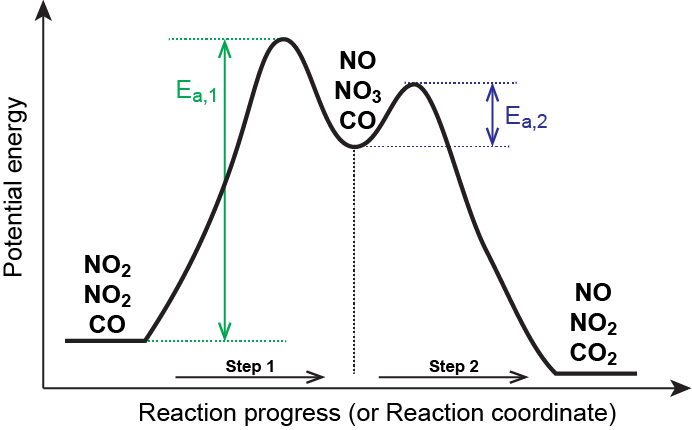
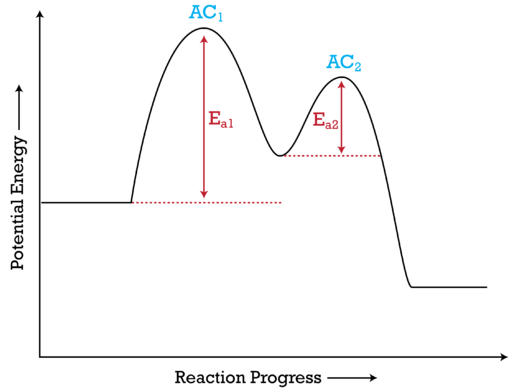
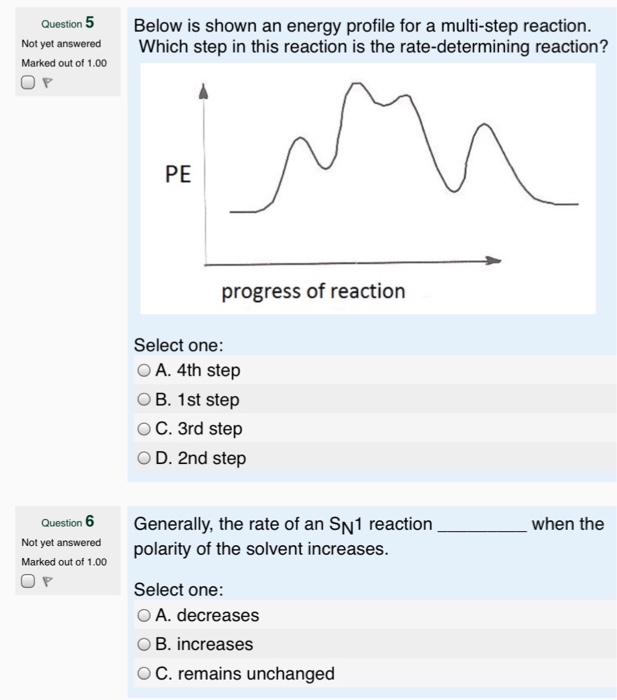
wisc.pb.unizin.org › chem103and104 › chapterM13Q10: Mechanisms and Multistep Reactions; Reaction Profiles ...... In a reaction coordinate diagram, each step in a multi-step mechanism will have its own activation energy, resulting in an energy peak for each step. The rate-determining, or slow, step will have the largest activation energy. This can be seen in Figure 2 below. Figure 2. study.com › skill › learnAnalyzing Multi-step Reaction Energy Profiles | Chemistry |... Steps for Analyzing Multi-step Reaction Energy Profiles Step 1: Count the number of low points and high points in the diagram. Step 2: Correlate the low points to intermediates and the high... › explanations › chemistryMultistep Reaction: Definition & Energy Profile | StudySmarter A multistep reaction is a series of reactions that can be "summed up" by a net reaction. The reaction is made up of several steps called elementary reactions and may involve a catalyst. The rate-determining step is the step that is the slowest (has the highest activation energy ).
Multistep reaction energy profile. study.com › skill › practiceAnalyzing Multi-step Reaction Energy Profiles - Study.com Analyzing Multi-step Reaction Energy Profiles High School Chemistry Skills Practice 1. Considering the graph, how many elementary steps are in the reaction mechanism? 2. On the following... › explanations › chemistryMultistep Reaction: Definition & Energy Profile | StudySmarter A multistep reaction is a series of reactions that can be "summed up" by a net reaction. The reaction is made up of several steps called elementary reactions and may involve a catalyst. The rate-determining step is the step that is the slowest (has the highest activation energy ). study.com › skill › learnAnalyzing Multi-step Reaction Energy Profiles | Chemistry |... Steps for Analyzing Multi-step Reaction Energy Profiles Step 1: Count the number of low points and high points in the diagram. Step 2: Correlate the low points to intermediates and the high... wisc.pb.unizin.org › chem103and104 › chapterM13Q10: Mechanisms and Multistep Reactions; Reaction Profiles ...... In a reaction coordinate diagram, each step in a multi-step mechanism will have its own activation energy, resulting in an energy peak for each step. The rate-determining, or slow, step will have the largest activation energy. This can be seen in Figure 2 below. Figure 2.























Komentar
Posting Komentar